7 Expert-Approved Ways to Avoid A Blood Sugar Spike
페이지 정보
작성자 Dalton 작성일25-09-22 14:02 조회12회 댓글0건관련링크
본문
 Id. Further, even if the proof supporting the claim was qualitatively weaker than the evidence towards it, FDA failed to supply "empirical proof" that an applicable disclaimer would confuse shoppers and fail to appropriate for deceptiveness. In the file before it, the District Court found, "some evidence" (248 F. supp. On August 12, 2002, FDA’s Minneapolis District Office issued a Warning Letter to the Conklin Company, Inc., Shakopee, Minnesota. FDA’s Los Angeles District Office performed an inspection of TriMedica International, Inc., Tempe, Arizona, on May 22 - 23, 2002, as a observe-as much as a client complaint. An FDA inspection of the agency on April 24 - 25, 2001, June 5 - 6, 2002, and July 8, 2002, disclosed violations of the Federal Food, Drug, and Cosmetic Act. The task Force additionally suggests that FDA request feedback on how the agency could greatest educate shoppers concerning the position of qualified well being claims on food labeling, and the way such claims could also be utilized by consumers to advance their very own understanding of food regimen and well being matters. As these efforts transfer into extra in-depth understanding and increasingly rising science, as well as increase to encompass an array of stakeholders, they must be encouraged and promoted by use of the meals label.
Id. Further, even if the proof supporting the claim was qualitatively weaker than the evidence towards it, FDA failed to supply "empirical proof" that an applicable disclaimer would confuse shoppers and fail to appropriate for deceptiveness. In the file before it, the District Court found, "some evidence" (248 F. supp. On August 12, 2002, FDA’s Minneapolis District Office issued a Warning Letter to the Conklin Company, Inc., Shakopee, Minnesota. FDA’s Los Angeles District Office performed an inspection of TriMedica International, Inc., Tempe, Arizona, on May 22 - 23, 2002, as a observe-as much as a client complaint. An FDA inspection of the agency on April 24 - 25, 2001, June 5 - 6, 2002, and July 8, 2002, disclosed violations of the Federal Food, Drug, and Cosmetic Act. The task Force additionally suggests that FDA request feedback on how the agency could greatest educate shoppers concerning the position of qualified well being claims on food labeling, and the way such claims could also be utilized by consumers to advance their very own understanding of food regimen and well being matters. As these efforts transfer into extra in-depth understanding and increasingly rising science, as well as increase to encompass an array of stakeholders, they must be encouraged and promoted by use of the meals label.
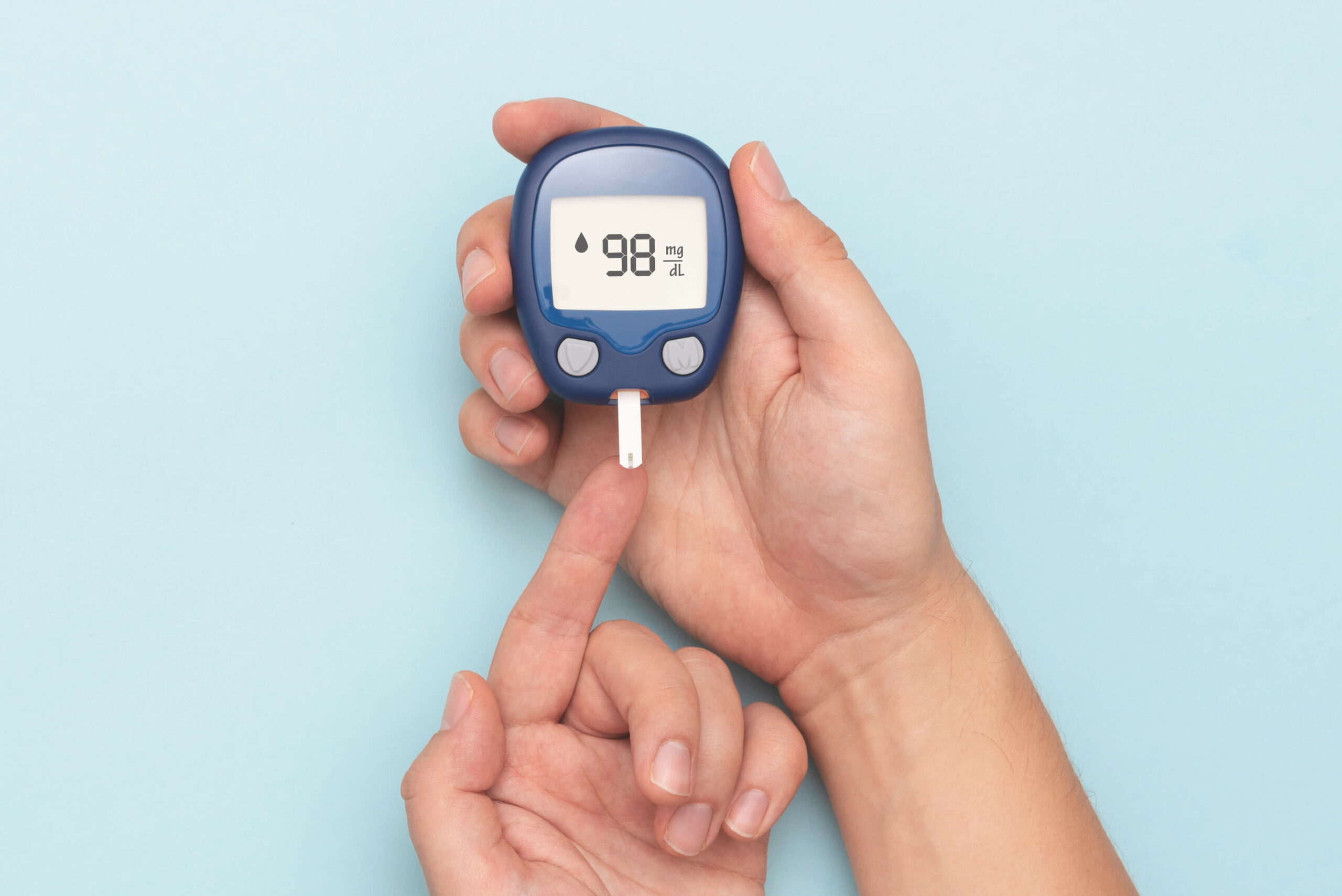
 Understanding the consequences of different foods on high Gluco Shield Blood Support reviews sugar can empower you to make better dietary selections. The American Society of Anesthesiologists and Stanford University School of Medicine's Department of Otolaryngology specifically recommend avoiding vitamin E earlier than surgical procedure as a result of it could increase the chance of bleeding and trigger issues along with your blood pressure. Several different merchandise (Life Track Vitamin E, Multi Mineral, Vitamin C, Vitamin B-Complex, Multi Vitamin and Bone Support) are misbranded because they fail to bear the Supplement Facts Panel. Id. Thus, under the District Court's view of Pearson, FDA may have imposed an outright ban on a well being declare solely where there is either "no proof" to assist a claim or where the proof for a declare is "qualitatively weaker than the proof towards the declare -- for instance, the place the claim rests on only one or two previous studies." Id., quoting Pearson, 164 F.3d at 659-660 and n.10 (emphasis in authentic). Similar results have been found in adults. Normally, the potential side effects of taking berberine are thought to be minimal.
Understanding the consequences of different foods on high Gluco Shield Blood Support reviews sugar can empower you to make better dietary selections. The American Society of Anesthesiologists and Stanford University School of Medicine's Department of Otolaryngology specifically recommend avoiding vitamin E earlier than surgical procedure as a result of it could increase the chance of bleeding and trigger issues along with your blood pressure. Several different merchandise (Life Track Vitamin E, Multi Mineral, Vitamin C, Vitamin B-Complex, Multi Vitamin and Bone Support) are misbranded because they fail to bear the Supplement Facts Panel. Id. Thus, under the District Court's view of Pearson, FDA may have imposed an outright ban on a well being declare solely where there is either "no proof" to assist a claim or where the proof for a declare is "qualitatively weaker than the proof towards the declare -- for instance, the place the claim rests on only one or two previous studies." Id., quoting Pearson, 164 F.3d at 659-660 and n.10 (emphasis in authentic). Similar results have been found in adults. Normally, the potential side effects of taking berberine are thought to be minimal.
LOL, she nonetheless has chores, homework, caring for her canine, life goes on. In a research by Lendon Smith, MD, of 6000 hyperactive youngsters, 80% confirmed improvement within a couple of weeks by simply altering their weight loss plan and dietary blood sugar balance supplement sugar supplement taking nutritional supplements. For decades, FDA regulated dietary supplements as foods, in most circumstances, to ensure that they had been secure and wholesome, Gluco Shield Blood Support reviews and that their labeling was truthful and not misleading. To this end, the task Force encourages FDA to work with sister agencies and stakeholders to determine one of the best, most applicable, and most useful dietary steering statements for customers, and in turn present for simple and meaningful ways that such data can seem in labeling. The duty Force recommends that FDA seek alternatives to train flexibility in its analysis of well being declare petitions in these and different areas the place feasible, and that the agency also seek alternatives to promote the development and use of extra dietary guidance statements on foods in order that the US population might be encouraged to make better meals decisions and set up healthier consuming patterns by making healthier meals selections. However, when there's a public well being benefit FDA has made exceptions to those disqualifying levels. There aren't any recombinant hGH products which might be authorised by FDA for anti-aging treatment.
There may be interest in expanding the appliance of qualified well being claims to areas beyond these related to uncertain science. The uses promoted for the drug included claims akin to "decrease in fat, increase in muscle, improved skin texture, lower in wrinkles, increased immunity, higher sleep and increased cardiac output and kidney function." This classifies the product as a "new drug" without an accredited New Drug Application. Your hGH product is being promoted and distributed for an unapproved use. The agency manufactures varied products promoted as dietary supplements. Because dietary supplements are below the "umbrella" of foods, FDA's Center for Food Safety and Applied Nutrition (CFSAN) is chargeable for the agency's oversight of these merchandise. Because of this of those provisions, dietary components utilized in dietary supplements are no longer topic to the premarket security evaluations required of other new meals substances or for brand new uses of old meals components. However, with passage of the Dietary Supplements Health and Education Act of 1994 (DSHEA), Congress amended the Act to incorporate several provisions that apply only to dietary supplements and dietary components of dietary supplements.
댓글목록
등록된 댓글이 없습니다.
